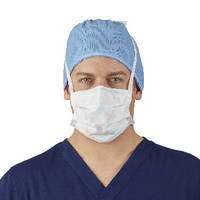
Surgical Mask
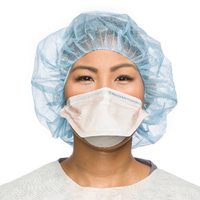
N95 Respirator
Approval and Regulations
Cleared by the U.S. Food and Drug Administration (FDA) and regulated under 21 CFR 878.4040.
Regulated by the FDA, CDC NIOSH, OSHA. Evaluated, tested, and approved by NIOSH as per the requirements in 42 CFR Part 84.
Intended Purpose
Fluid resistant. Reduces wearer’s exposure to and provides protection against large droplets, splashes, or sprays of bodily or other hazardous fluids. Protects the patient from the wearer’s respiratory emissions.
Reduces wearer’s exposure to particles including small particle aerosols and large droplets (only non-oil aerosols). Designed to protect both the patient and healthcare professional from the transfer of microorganisms, bodily fluids and particulate material at an N95 filtration level per 42 CFR 84.181.
Use Limitations
Disposable. Not intended to be used more than once and should be discarded if damaged, soiled or if breathing through the mask becomes difficult.
Ideally should be discarded after each patient or customer encounter. It should also be discarded when it becomes damaged or deformed; no longer forms and effective seal to the face; becomes wet or visibly dirty; breathing becomes difficult, or it becomes contaminated with any fluids from patients or customers.
Filtration
Not considered respiratory protection. Does NOT provide the wearer with a reliable level of protection from inhaling smaller airborne particles.
Is considered respiratory protection. Filters out at least 95% of airborne particles including large and small particles.
Air Leakage
Leakage occurs around the edge of the mask when user inhales.
When properly fitted and donned donned and fitted, minimal leakage occurs around edges of the respirator when user inhales.
Fit Testing Requirement
Not required
Yes, per OSHA standard 1910.134 App A
User Seal Check Requirement
Not required
Yes. Required each time the respirator is put on, known as donning.
Related Products
Sources:
1. https://www.cdc.gov/niosh/npptl/pdfs/UnderstandDifferenceInfographic-508.pdf (Retrieved May 12, 2021)
2. https://www.fda.gov/medical-devices/personal-protective-equipment-infection-control/n95-respirators-surgical-masks-and-face-masks (Retrieved May 12, 2021)
3. https://www.fda.gov/about-fda/domestic-mous/mou-225-18-006 (Retrieved May 12, 2021)
